Reliable, rapid chromatographic immunoassay for the qualitative,
detection of specific antigens of SARS-CoV-2 present
in the human nasopharynx
SARS-CoV-2
Rapid Antigen Test
SARS-CoV-2 RT-PCR Detection Kit
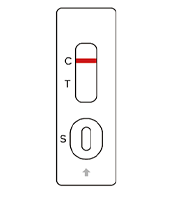
SARS-COV-2
Negative
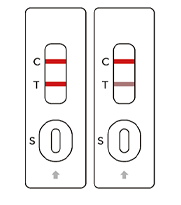
SARS-COV-2
Positive
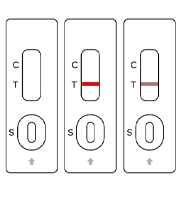
SARS-COV-2
Invalid
Material Provided
10 sterile swabs
10 extraction Tubes
10 dropper tips for extraction tubes
1 buffer solution (0,09 % sodium azide), sufficient for 10 tests
1 workstation
1 instruction manual (IFU)
Warnings and Precautions
1. For in vitro diagnostic use only.
2. The test device should remain in the sealed pouch until use.
3. Do not use kit past its expiration date.
4. Swabs, tubes and test devices are for single use only.
5. Do not interchange or mix components from different kit lots.
6. Testing should only be performed using the swabs provided within the kit.
7. To obtain accurate results, do not use visually bloody or overly viscous samples.
8. If the test is carried out by or being supervised by a healthcare professional or trained individual, it is recommended they wear appropriate personal protection equipment (PPE), whist changing gloves between patients. The patients themselves does not need to wear PPE.
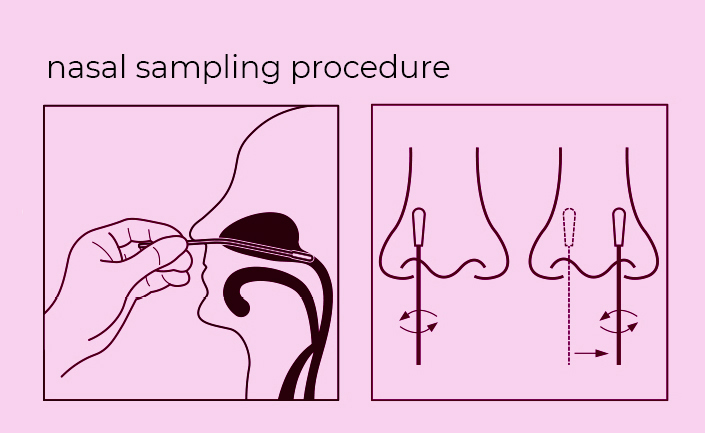
9. Specimens must be processed as indicated in the SPECIMEN COLLECTION and SAMPLE PREPARATION PROCEDURE sections of this Product Insert. Failure to follow the instructions for use can result in inaccurate results.
10. Proper laboratory safety techniques should be followed at all times when working with SARS-CoV-2 patient samples. Patient swabs, used Test Strips and used extraction buffer vials may be potentially infectious. Proper handling and disposal methods should be established by the laboratory in accordance with local regulatory requirements.
11. Inadequate or inappropriate specimen collection and storage can adversely affect results.
12. Humidity and temperature can adversely affect results.
13. Dispose of test device and materials
Steps for Simple Test
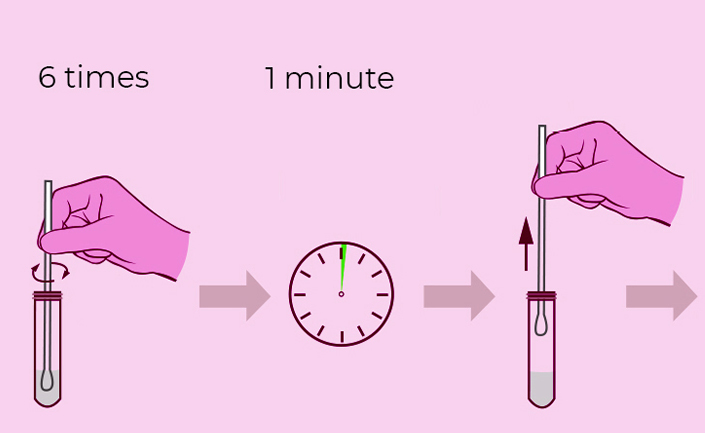
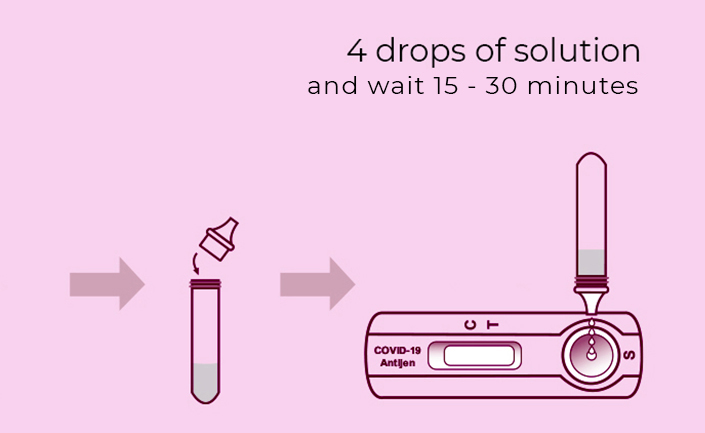
**Warning**
NOTE: Test results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA. Clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
